In order to fully exploit the potential of green hydrogen production in an experimental setting, GUNT offers a system consisting of coordinated experimental components:
- Photovoltaic modules ET 255.02 and Wind power plant ET 255.04 as renewable energy sources
- Energy system ET 255 for optimised self-consumption through storage utilisation with energy management system
- AEM electrolyser ET 280 for hydrogen production

ET 255.01 Photovoltaic simulator
- simulation of current-voltage characteristics of photovoltaic modules
- time-controlled specification of generation and consumption profiles
ET 255.02 Photovoltaic modules
- mobile pivoting frame with 4 photovoltaic modules, adjustable angle of inclination
- sensors for module temperature and illuminance
ET 255.04 Wind power plant
ET 255.04 serves as an additional renewable energy source.
- emergency stop switch as a safety device
ET 255 Energy system for solar and wind power
ET 255 contains all necessary networked components of an energy system.
- generator connection box with circuit breaker and overvoltage protection as a safety device
- charge controller for power optimisation (MPP tracker)
- LiFePO accumulator with battery management system
- inverter for grid feed-in and stand-alone operation
- bidirectional electricity meter with communication interface
- grid connection
ET 280 Modular electrolyser for H2 (AEM)
ET 280 contains an electrolyser with a stack consisting of several series-connected cells in bipolar design.
- reverse osmosis system for water purification
- air-cooled electrolyser with anion exchange membrane (AEM)
- gas burner for flaring the generated hydrogen
Hydrogen production through AEM electrolysis using renewable energy
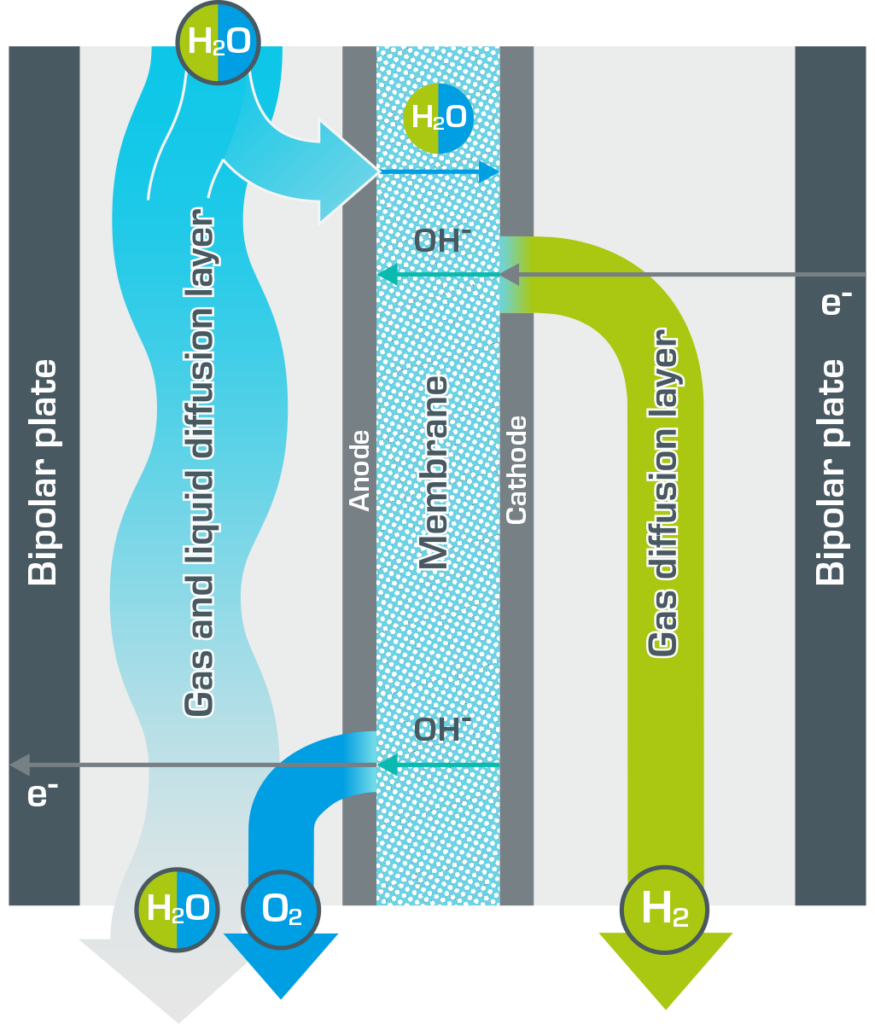
Information on the operating principle
The Anion Exchange Membrane (AEM) divides the AEM electrolyser into two half-cells. An aqueous potassium hydroxide solution circulates as the electrolyte in the anode half-cell, saturating the membrane. There is no liquid in the cathode half-cell. Water passes through the membrane and is reduced at the cathode.
4H2O + 4e– → 4OH– + 2H2
The hydrogen produced escapes, while the hydroxide ions migrate back into the anode half-cell. This produces oxygen and water at the anode.
4OH–→ 2H2O + O2 + 4e–
